Abstract
Introduction
To prevent CAR-T fratricide, anti-CD7 CAR (7CAR) T cells used for treating T-cell malignancies are often modified by CD7 ablation via CRISPR/CAS9 gene editing or by co-expression of a CD7-specific protein expression blocker. Both methods require additional genetic manipulations of CAR-T. Here we transduce 7CAR into bulk T cells without CD7 disruption and thereafter allow CAR-T cells to emerge in vitro after fratricidal "natural selection". The biological characteristics of these naturally selected anti-CD7 CAR (NS7CAR) T cells and their potential advantages in treating patients with T-cell malignancies are described.
Methods
The percentage of CD3 +CD7 - T cells in peripheral blood from either healthy donors (HDs) or patients (PTs) were determined by flow cytometry. Peripheral bulk T cells were positively selected using CD3 magnetic beads, and peripheral CD7 - T cells were negatively selected using CD7 magnetic beads. To avoid contamination from malignant T cells, patients only with CD3 -CD7 + T cell blasts were included in this study. The 7CAR gene cassette comprising of the cDNA of a CD7-specific antibody sequence fused to the coding sequences for the CD8TM-41BB-CD3z signal domains, and the T2A-linked tEGFR was cloned into a lentiviral vector backbone under the control of an EF1α promoter. 7CAR lentiviral transduction of bulk T cells (NS7CAR) or CD7 - T cells (Neg7CAR) were performed two days after CD3/CD28 dynabeads activation. CD7-ablated 7CAR T cells (KO7CAR) were derived by electroporation of bulk T cells with CD7-targeting Cas9-gRNA RNP 24 hours before 7CAR transduction. CAR-T cells were routinely kept in culture for 12 days. The levels of CD7 mRNA, protein, and surface expression were determined respectively by qualitative/quantitative reverse transcription PCR, Western blotting, and flow cytometry. Iv vitro cytotoxic activity for CD7 + tumor cell lines was tested using a flow-cytometry-based cytotoxicity assay. NSG mice engrafted with CCRF-CEM-luciferase cells were used as an animal model to validate the activities of CD7 CAR.
Results
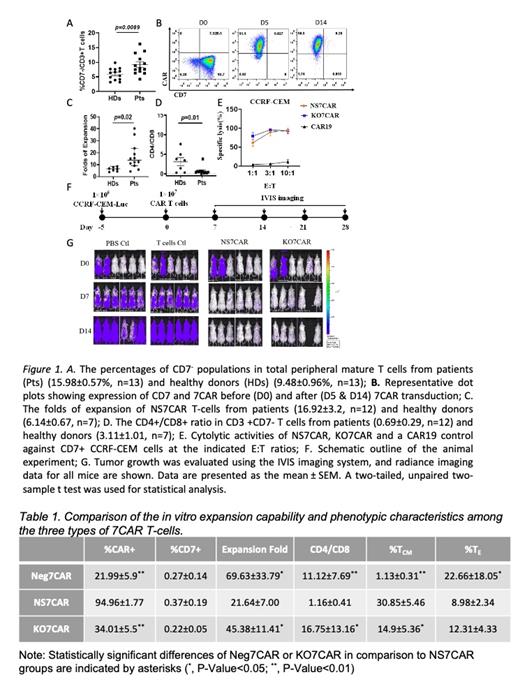
Three approaches for generating anti-CD7 CAR-T cells were compared: NS7CAR (fratricidal natural selection from bulk T cells after 7CAR transduction), Neg7CAR (7CAR transduction of purified CD7-negative T cells) and KO7CAR (Cas9 RNP CD7 gene ablation). While CD7 - T cells were detectable in HDs of all ages (9.48±0.96%, n=13), we observed a significant increase of this cell population in T-cell acute lymphoblastic leukemia PTs (15.98±0.57%, n=13) (Fig 1A). We next tested the feasibility of using bulk T cells to generate naturally occurring 7CAR T cells without CD7 gene ablation or protein blockage. Three days after 7CAR lentiviral transduction, purified bulk peripheral T cells had a rapid and dramatic phenotypic transition from CD7 + CAR - to CD7 -CAR +(Fig 1B). Although fratricide led to a much lower expansion and viability of 7CAR T cells compared to T- cells without 7CAR transduction, approximately 80% of the 7CAR T cells were viable, making further studies feasible. After 12 days of culture, 7CAR T cells from PTs displayed stronger expansion potential (Fig 1C) and contained a larger CD8 + subpopulation (Fig 1D) as compared to cells derived from HDs. In comparison to Neg7CAR and KO7CAR, the final NS7CAR product displayed a lower expansion capability, but contained a higher percentage of CAR + cells, a larger CD8 +subset and an increased central memory phenotype (Table 2). Interestingly, although the final cells from all three products had no surface CD7 expression, mRNA and total protein were only detected from NS7CAR, but not from Neg7CAR or KO7CAR. Additionally, NS7CAR showed superior cytotoxicity and cytokine release in an in vitro functional test (Fig 1E). In the animal model, the NS7CAR conferred robust protection against leukemia progression with marked reduction in leukemia cell burden in the first two weeks after CAR T- cells injection (Fig 1F&G).
Conclusion
Among the three approaches, the NS7CAR T cells was significantly enriched in CAR + cells and contained a higher percentage of CD8 + central memory T cells. Importantly, our data indicate that autologous PBMCs from patients were superior to PBMCs of healthy donors in yielding sufficient NS7CAR T cells for therapeutic needs. An investigator-initiated trial is currently ongoing to test the feasibility, efficacy, and safety of NS7CAR T cells for treating T-cell acute lymphoblastic leukemia.
Liu: SenlangBio: Current Employment. Ba: SenlangBio: Current Employment. Li: SenlangBio: Current holder of individual stocks in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal